RENMIN UNIVERSITY of CHINA



Recently, Professor Wang Yapei's research group from the School of Chemistry and Life Resources, Renmin University of China (RUC), achieved a significant breakthrough in the field of wireless optogenetic neural modulation. The research paper, titled "NIR-II Light-Activated Neurostimulation Enabled by Broad-Angle Light Concentrating", was published in Advanced Materials (DOI: 10.1002/adma.202522252).
The paper was co–first-authored by Zhang Nan, a PhD candidate at RUC, and Lyu Shanzhi, a postdoctoral researcher at Tsinghua University, with Wang Yapei as the corresponding author.
Implantable neural modulation systems are key technologies for treating neurological disorders such as Parkinson's disease and epilepsy. However, existing devices typically rely on bulky battery packs that require surgical replacement, severely limiting device miniaturization and long-term application. While emerging wireless light-based neural stimulation technologies show promise, they face major challenges in clinical use due to severe light scattering in biological tissues and the difficulty of precisely aligning external light sources with implanted devices, compromising reliability and precision.
To address these challenges, the research team drew inspiration from the highly efficient light-concentrating microstructures found in the petals of the Papaver radicatum. Based on this biological prototype, they designed an innovative lens-free broad-angle light concentrating (BAQC) layer. This biomimetic structure effectively concentrates non-collimated light propagating through biological tissue and is insensitive to the angle of incidence, overcoming key limitations of traditional lens-based systems in vivo.
In parallel, the team developed a photothermal–pyroelectric (PT–PE) energy conversion layer, leveraging the deep tissue penetration and high biosafety of near-infrared II (NIR-II, 1064 nm) light to efficiently convert optical energy into electrical pulses for neural stimulation.
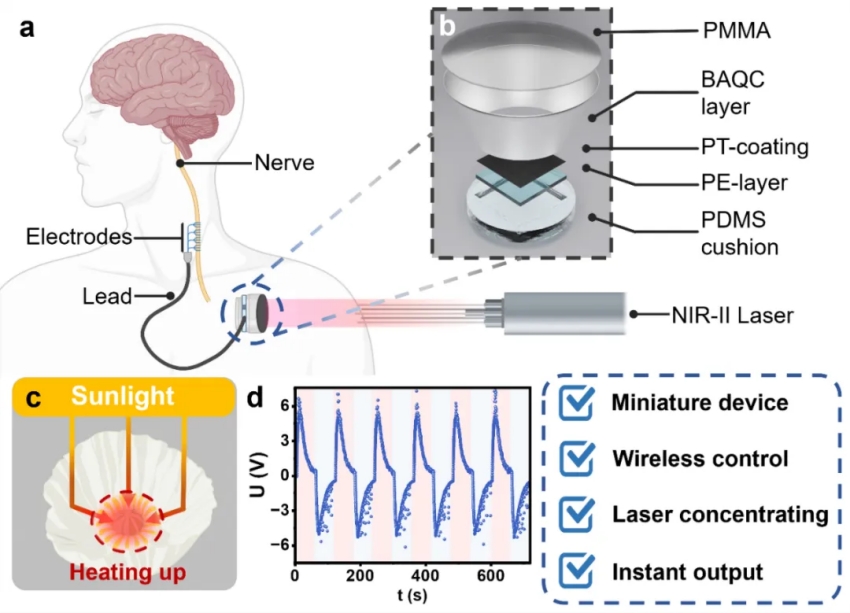
Experimental results show that the neural modulation device integrating the BAQC and PT–PE layers achieved a 214.5% improvement in light concentration efficiency compared to planar structures, while its overall volume was reduced to one-third of that of conventional lens-based systems, enabling substantial miniaturization. The device functioned reliably in standard electrical circuits and successfully activated the sciatic nerve of frogs, inducing repeatable, light-controlled contractions of the gastrocnemius muscle.
This study establishes a new paradigm for battery-free, wirelessly operated neural modulation that is insensitive to angular misalignment. The biomimetic light-concentrating strategy and photothermal–pyroelectric energy conversion mechanism developed in this work provide valuable insights for next-generation miniaturized, high-precision implantable bioelectronic systems, with broad application prospects in neural repair, brain–computer interfaces, and other frontier biomedical fields.